Pre-clinical evaluation of a next-generation spray adhesion barrier for multiple site adhesion protection
Surg Technol Int. 2009;18:137-43.
Pre-clinical evaluation of a next-generation spray adhesion barrier for multiple site adhesion protection.
Brown University, Providence, RI.
Abstract
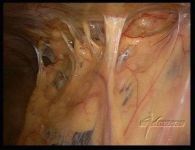
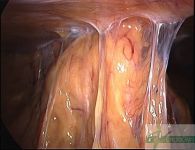
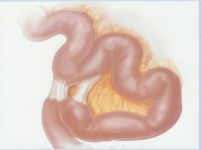
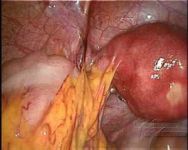
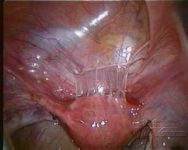
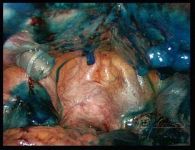
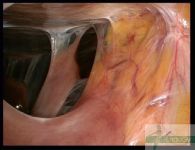
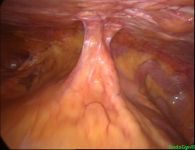
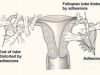
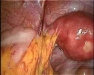
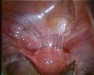
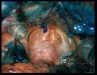
Intra-abdominal adhesions represent a major cause of postoperative morbidity, including chronic or recurrent pelvic pain and infertility in a significant percent of patients. The SprayShield Adhesion Barrier System (Covidien, Waltham, MA) is a next-generation sprayable adhesion barrier to prevent postoperative adhesions. Initially sprayed as a liquid, SprayShield solidifies within 2 seconds of contact with tissue through a polyethylene glycol (PEG) ester-Trilysine reaction to form an adherent, internal tissue barrier that protects the underlying tissues for several days after surgery. After tissue begins to heal, the adhesion barrier liquefies via hydrolysis and completely absorbs within 7 days. Safety testing has shown the product to be nongenotoxic, noncytotoxic, nonsensitizing, and nonirritating. SprayShield has been shown to adhere well to tissue, with the mechanism of adherence believed to be mainly due to tissue surface mechanical interlocking. In studies that compared SprayShield to good surgical technique, virgin hogs were randomized to receive SprayShield or good surgical technique (Control). Compared to Controls, SprayShield demonstrated a statistically significant reduction in the number of adhesions (46%, p=0.04) and in the area of adhesions (83%, p=0.012) to injured sites. With its ease of application, biocompatibility and adhesion prevention efficacy, SprayShield may be an effective next-generation adhesion prevention product for open and laparoscopic abdominopelvic procedures as an adjunct to good surgical technique.